Stability of Beryllium and Carbon
About points...
We associate a certain number of points with each exercise.
When you click an exercise into a collection, this number will be taken as points for the exercise, kind of "by default".
But once the exercise is on the collection, you can edit the number of points for the exercise in the collection independently, without any effect on "points by default" as represented by the number here.
That being said... How many "default points" should you associate with an exercise upon creation?
As with difficulty, there is no straight forward and generally accepted way.
But as a guideline, we tend to give as many points by default as there are mathematical steps to do in the exercise.
Again, very vague... But the number should kind of represent the "work" required.
When you click an exercise into a collection, this number will be taken as points for the exercise, kind of "by default".
But once the exercise is on the collection, you can edit the number of points for the exercise in the collection independently, without any effect on "points by default" as represented by the number here.
That being said... How many "default points" should you associate with an exercise upon creation?
As with difficulty, there is no straight forward and generally accepted way.
But as a guideline, we tend to give as many points by default as there are mathematical steps to do in the exercise.
Again, very vague... But the number should kind of represent the "work" required.
About difficulty...
We associate a certain difficulty with each exercise.
When you click an exercise into a collection, this number will be taken as difficulty for the exercise, kind of "by default".
But once the exercise is on the collection, you can edit its difficulty in the collection independently, without any effect on the "difficulty by default" here.
Why we use chess pieces? Well... we like chess, we like playing around with \(\LaTeX\)-fonts, we wanted symbols that need less space than six stars in a table-column... But in your layouts, you are of course free to indicate the difficulty of the exercise the way you want.
That being said... How "difficult" is an exercise? It depends on many factors, like what was being taught etc.
In physics exercises, we try to follow this pattern:
Level 1 - One formula (one you would find in a reference book) is enough to solve the exercise. Example exercise
Level 2 - Two formulas are needed, it's possible to compute an "in-between" solution, i.e. no algebraic equation needed. Example exercise
Level 3 - "Chain-computations" like on level 2, but 3+ calculations. Still, no equations, i.e. you are not forced to solve it in an algebraic manner. Example exercise
Level 4 - Exercise needs to be solved by algebraic equations, not possible to calculate numerical "in-between" results. Example exercise
Level 5 -
Level 6 -
When you click an exercise into a collection, this number will be taken as difficulty for the exercise, kind of "by default".
But once the exercise is on the collection, you can edit its difficulty in the collection independently, without any effect on the "difficulty by default" here.
Why we use chess pieces? Well... we like chess, we like playing around with \(\LaTeX\)-fonts, we wanted symbols that need less space than six stars in a table-column... But in your layouts, you are of course free to indicate the difficulty of the exercise the way you want.
That being said... How "difficult" is an exercise? It depends on many factors, like what was being taught etc.
In physics exercises, we try to follow this pattern:
Level 1 - One formula (one you would find in a reference book) is enough to solve the exercise. Example exercise
Level 2 - Two formulas are needed, it's possible to compute an "in-between" solution, i.e. no algebraic equation needed. Example exercise
Level 3 - "Chain-computations" like on level 2, but 3+ calculations. Still, no equations, i.e. you are not forced to solve it in an algebraic manner. Example exercise
Level 4 - Exercise needs to be solved by algebraic equations, not possible to calculate numerical "in-between" results. Example exercise
Level 5 -
Level 6 -
Question
Solution
Short
Video
\(\LaTeX\)

Need help? Yes, please!
The following quantities appear in the problem:
The following formulas must be used to solve the exercise:

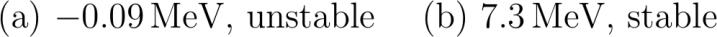
No explanation / solution video to this exercise has yet been created.
Visit our YouTube-Channel to see solutions to other exercises.
Don't forget to subscribe to our channel, like the videos and leave comments!
Visit our YouTube-Channel to see solutions to other exercises.
Don't forget to subscribe to our channel, like the videos and leave comments!
Exercise:
vspacmm abcliste abc Show that the nucleus isotopeBe .u is unstable and will decay o two upalpha particles .u. abc Is isotopeC .u by definition stable against decay o three upalpha particles? Show why or why not. abcliste
Solution:
abcliste abc The mass difference between left and right is: Delta m MisotopeBe - MisotopeHe .u - .u .u Because the massdifference is positive i.e. the left side is heavier than the right side it means mass got lost in the reaction i.e. energy was produced i.e. the reaction ts to happen spontaneous i.e. Beryllium is unstable. abc The binding enregy of this Carbon isotope considered consisting of Helium atoms is: Delta m MisotopeC - MisotopeHe .u - .u -.u Because the massdifference is negative i.e. the left side is lighter than the right side it means mass got produced in the reaction i.e. energy is needed i.e. the reaction can't happen spontaneous i.e. Carbon is stable. abcliste
vspacmm abcliste abc Show that the nucleus isotopeBe .u is unstable and will decay o two upalpha particles .u. abc Is isotopeC .u by definition stable against decay o three upalpha particles? Show why or why not. abcliste
Solution:
abcliste abc The mass difference between left and right is: Delta m MisotopeBe - MisotopeHe .u - .u .u Because the massdifference is positive i.e. the left side is heavier than the right side it means mass got lost in the reaction i.e. energy was produced i.e. the reaction ts to happen spontaneous i.e. Beryllium is unstable. abc The binding enregy of this Carbon isotope considered consisting of Helium atoms is: Delta m MisotopeC - MisotopeHe .u - .u -.u Because the massdifference is negative i.e. the left side is lighter than the right side it means mass got produced in the reaction i.e. energy is needed i.e. the reaction can't happen spontaneous i.e. Carbon is stable. abcliste
Meta Information
Exercise:
vspacmm abcliste abc Show that the nucleus isotopeBe .u is unstable and will decay o two upalpha particles .u. abc Is isotopeC .u by definition stable against decay o three upalpha particles? Show why or why not. abcliste
Solution:
abcliste abc The mass difference between left and right is: Delta m MisotopeBe - MisotopeHe .u - .u .u Because the massdifference is positive i.e. the left side is heavier than the right side it means mass got lost in the reaction i.e. energy was produced i.e. the reaction ts to happen spontaneous i.e. Beryllium is unstable. abc The binding enregy of this Carbon isotope considered consisting of Helium atoms is: Delta m MisotopeC - MisotopeHe .u - .u -.u Because the massdifference is negative i.e. the left side is lighter than the right side it means mass got produced in the reaction i.e. energy is needed i.e. the reaction can't happen spontaneous i.e. Carbon is stable. abcliste
vspacmm abcliste abc Show that the nucleus isotopeBe .u is unstable and will decay o two upalpha particles .u. abc Is isotopeC .u by definition stable against decay o three upalpha particles? Show why or why not. abcliste
Solution:
abcliste abc The mass difference between left and right is: Delta m MisotopeBe - MisotopeHe .u - .u .u Because the massdifference is positive i.e. the left side is heavier than the right side it means mass got lost in the reaction i.e. energy was produced i.e. the reaction ts to happen spontaneous i.e. Beryllium is unstable. abc The binding enregy of this Carbon isotope considered consisting of Helium atoms is: Delta m MisotopeC - MisotopeHe .u - .u -.u Because the massdifference is negative i.e. the left side is lighter than the right side it means mass got produced in the reaction i.e. energy is needed i.e. the reaction can't happen spontaneous i.e. Carbon is stable. abcliste
Contained in these collections:
-
Kernreaktionen by uz
-
Spontan mögliche Kernreaktionen by TeXercises